Abstract
Background: Follicular lymphoma (FL) is the second most common form of non-Hodgkin lymphoma (NHL) in Western countries, accounting for 20-30% of all NHLs (Hübel K. Hemasphere. 2020;4:e317). While most patients (pts) respond well to first-line therapy, they typically experience frequent relapses and progressively shorter duration of response with subsequent lines of therapy (Batlevi CL. Blood Cancer J. 2020;10:74; Rivas-Delgado A. Br J Haematol. 2019;184:753-9), and increasingly refractory disease with limited treatment options. Thus, there is an unmet need for effective treatment options for pts with relapsed or refractory (R/R) FL. Parsaclisib is a potent, highly selective, next-generation phosphatidylinositol 3-kinase (PI3K)δ inhibitor. Here we report results of the primary analysis of CITADEL-203 (NCT03126019, EudraCT 2017-001624-22), a phase 2, multicenter, open-label study of parsaclisib monotherapy in R/R FL.
Methods: Eligible pts were ≥18 years of age, had histologically confirmed R/R FL (grade 1, 2, or 3a), had received ≥2 prior systemic therapies (not including PI3K inhibitors or Bruton's kinase inhibitors), had an Eastern Cooperative Oncology Group performance status (ECOG PS) ≤2, and were ineligible for hematopoietic stem cell therapy. Pts were allocated to receive 20 mg parsaclisib once daily (QD) for 8 weeks, followed by parsaclisib either 20 mg once weekly (weekly-dosing group [WG]) or 2.5 mg QD (daily-dosing group [DG]). Prophylaxis for Pneumocystis jirovecii pneumonia was required. The primary endpoint was objective response rate (ORR) as determined by an independent review committee (IRC); secondary endpoints included complete response rate (CRR), duration of response (DOR), progression-free survival (PFS), overall survival (OS), and safety and tolerability. All radiology-based endpoints were confirmed by an IRC.
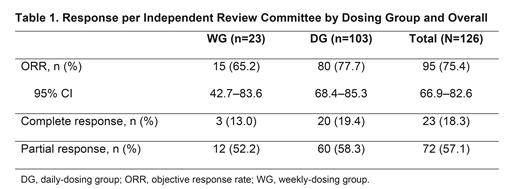
Results: At data cutoff for the primary analysis (Jan 15, 2021), 126 pts (WG, n=23; DG, n=103) had been treated. The median (range) age was 67.5 (40-88) years, 55.6% of pts were male, and majority (93.7%) of pts had an ECOG PS ≤1. The median (range) time since initial diagnosis was 5.95 (0.2-32.2) years. 54.0% of pts had received 2 lines and 27.8% had received 3 lines of prior systemic therapy (median [range], 2 [1-8]); 41.3% of pts had relapsed disease and 49.2% were refractory to their most recent prior therapy. 87 pts (69.0%) had discontinued treatment, primarily due to progressive disease (36.5%) or adverse events (21.4%). The median (range) treatment duration and follow-up from first dose to data cutoff were 8.5 (0.5-27.2) and 20.6 (5.7-34.1) months for all treated pts, and 8.4 (0.8-27.2) and 17.6 (5.7-33.1) months for the DG. The ORR (95% CI) was 75.4% (66.9-82.6) for all treated pts and 77.7% (68.4-85.3) for the DG (Table 1); CRR (95% CI) was 18.3% (11.9-26.1) for all pts and 19.4% (12.3-28.4) for the DG. Among all treated pts with complete or partial response, 73.7% of responses occurred at the first disease assessment. Median (95% CI) DOR was 14.7 (12.0-20.3) months for all pts and 14.7 (10.4-not estimable [NE]) months for the DG. Median (95% CI) PFS was 14.0 (11.3-19.6) months for all pts and 15.8 (11.0-NE) months for the DG. Median OS was not reached.
Among 126 treated pts, treatment-emergent adverse events (TEAEs) occurred in 97.6% (n=123) of pts (grade ≥3 in 58.7% [n=74]). The most common TEAEs were diarrhea (38.1%), nausea (24.6%), and cough (22.2%); most common grade ≥3 TEAEs included diarrhea (11.9%) and neutropenia (10.3%). TEAEs leading to dose interruption or dose reduction occurred in 46.8% and 17.5% of pts, respectively. TEAEs led to treatment discontinuation in 23.8% of all pts; the most common were diarrhea (7.1%), colitis (4.0%), pneumonitis, and rash (2.4% each). Serious TEAEs were experienced by 45.2% (n=57) of pts overall; the most common reported among all pts were diarrhea (7.1%), colitis (6.3%), and pneumonitis (2.4%). Two pts (1.6%) overall experienced a fatal TEAE.
Conclusions: Parsaclisib monotherapy demonstrated a rapid and durable response, had an acceptable safety profile, and was generally well tolerated in pts with R/R FL. These data suggest that parsaclisib could be a favorable treatment option for pts with R/R FL.
Lynch: Morphosys: Consultancy; Takeda: Research Funding; Incyte: Research Funding; TG Therapeutics: Research Funding; Rhizen: Research Funding; Bayer: Research Funding; Juno: Research Funding; Cyteir: Research Funding; Genentech: Research Funding. Avigdor: Pfizer: Consultancy, Honoraria; Gilead: Consultancy, Honoraria; BMS: Research Funding; Janssen: Research Funding; Takeda: Consultancy, Honoraria. McKinney: ADC Therapeutics: Consultancy, Speakers Bureau; Pharmacyclics: Consultancy; Novartis: Research Funding; Nordic Nanovector: Research Funding; Molecular Templates: Consultancy, Research Funding; Kite/Gilead: Honoraria, Speakers Bureau; Incyte: Research Funding; Genetech: Consultancy, Honoraria, Research Funding; Celgene: Consultancy, Research Funding; Epizyme: Consultancy; BTG: Consultancy; Beigene: Research Funding; Verastem: Consultancy. Paneesha: AbbVie: Honoraria; Bristol Myers Squibb: Honoraria; Gilead: Honoraria; Janssen: Honoraria; Roche: Honoraria; Celgene: Honoraria. Wahlin: Gilead Sciences: Research Funding; Roche: Consultancy, Research Funding. Cunningham: Celgene: Research Funding; OVIBIO: Membership on an entity's Board of Directors or advisory committees; Bayer: Research Funding; 4SC: Research Funding; Eli Lilly: Research Funding; Clovis Oncology: Research Funding; MedImmune: Research Funding; AstraZeneca: Research Funding; Roche: Research Funding. Morley: AbbVie; Takeda: Other: Conference support; Janssen: Honoraria; Kite: Honoraria; Roche: Membership on an entity's Board of Directors or advisory committees, Other: Conference support. Canales: Celgene/Bristol-Myers Squibb: Consultancy, Honoraria; Sandoz: Honoraria, Speakers Bureau; Sanofi: Consultancy; F. Hoffmann-La Roche Ltd: Consultancy, Honoraria, Speakers Bureau; Takeda: Consultancy, Honoraria, Speakers Bureau; Incyte: Consultancy; Gilead/Kite: Consultancy, Honoraria; Janssen: Consultancy, Honoraria, Speakers Bureau; Eusa Pharma: Consultancy, Honoraria; Karyopharm: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; iQone: Honoraria. Bastos-Oreiro: Takeda: Speakers Bureau; Novartis: Honoraria, Speakers Bureau; Kite: Speakers Bureau; Gilead: Honoraria; BMS-Celgene: Honoraria, Speakers Bureau; Janssen: Honoraria, Speakers Bureau; F. Hoffmann-La Roche: Honoraria, Research Funding, Speakers Bureau. Belada: Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel expenses, Research Funding; Celgene: Research Funding; Gilead: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel support, Research Funding; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Roche: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: travel expenses, Research Funding. Zheng: Incyte Corporation: Current Employment, Current equity holder in publicly-traded company. DeMarini: Incyte: Current Employment, Current equity holder in publicly-traded company. Jiang: Incyte: Current Employment, Current equity holder in publicly-traded company. Trněný: Gilead Sciences: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses; MorphoSys: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Bristol-Myers Squibb: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses; AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses; Incyte: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Honoraria; Amgen: Consultancy, Honoraria; 1st Faculty of Medicine, Charles University, General Hospital in Prague: Current Employment; Celgene: Consultancy; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses; Portola: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees.
Investigational PI3K delta inhibitor (parsaclisib) for patients with FL

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal